Good morning. Today, we’re talking about the plans to get New York open again (spoiler alert, you’re not seeing a Broadway show anytime soon) and a pharma CEO using Covid-19 clickbait to make himself $17 million. We’re also introducing a new regular section today: Investor Corner, where we feature a biopharma company that’s had a big week.
Waking up the city that never sleeps
The pulse:
Yesterday, Governor Cuomo rolled out some soft guidelines on what re-opening New York might look like. Drum roll please…
When will New York start to reopen?
New York’s stay-at-home order expires on the fast-approaching deadline of May 15th. After that, the city will start to re-open gradually, in four phases.
The phases:
Construction and manufacturing will be the first industries allowed to start up again.
If, after two weeks, infection rates seem stable, industries such as retail, real estate, and professional services may be allowed to open.
Restaurants, bars, and hotels come next.
Arts, entertainment, and recreation are the final phase. Schools are also grouped into this phase (we personally didn’t find high school to be that entertaining, though maybe Governor Cuomo disagrees) but it’s already been announced that schools will remain closed for the rest of the academic year.
Is New York really ready to re-open?
Are any of us really ready to put on real clothes every morning? As of right now, the answers to both these questions is no. New York has been the state hit hardest by Covid-19. Over 19,000 people have died from the coronavirus outbreak in New York, and 13,000 of those deaths have been in New York City.
However, New York has also been a leader in virus testing – nearly 1 in 7 tests in the U.S. have been done in NY, with more than 1,000,000 tests already performed in the state. Governor Cuomo has defined a strict list of eligibility criteria each region must meet before being allowed to re-open.
What are these re-opening criteria?
There are seven specific criteria, which include showing a decline in hospitalizations and Covid-19 related deaths over the span of 14 days, having at least 30% of hospital beds vacant, and ensuring that testing is performed at a rate of 30 weekly tests per 1,000 residents.
Bottom line it for me.
Currently, no region has met the eligibility criteria, but it’s more likely that less populated areas in the state will be able to open up first. Social distancing measures will be required regardless of the region or industry.
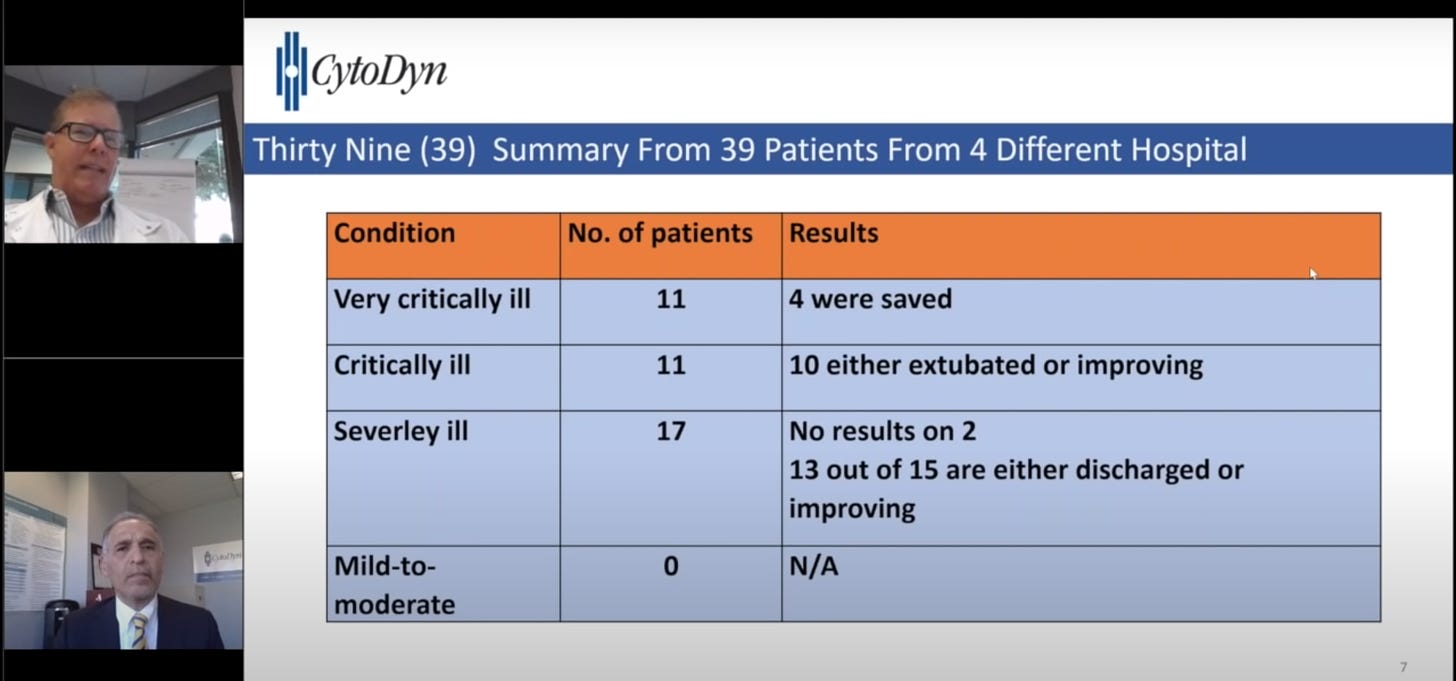
Source: A screenshot from CytoDyn’s 5/1 YouTube video discussing the efficacy of leronlimab.
Even pharma uses clickbait
The pulse:
The CEO of a small drug-maker, CytoDyn, filed to sell $17 million of company stock last Thursday, even as he pumped up stock prices by claiming that his company’s HIV drug helps save the lives of Covid-19 patients.
That sounds sketchy.
CEO Nader Pourhassan seems to operate by the motto “Do as I say not as I do.” He’s appeared in multiple YouTube videos since March citing leronlimab as a potential cure for the virus. In one of the videos, titled “CytoDyn leronlimab for COVID-19 coronavirus treatment. NEXT SUPER STOCK livestream 5/1/ 2020” he says:
The whole world is worried about the coronavirus, this is probably the best solution.
Meanwhile, leronlimab’s efficacy against the virus is uncertain. The drug was given to six Covid-19 patients in an April study, but physicians were unable to draw any conclusions about its safety or efficacy. There are no other published results in peer-reviewed journals on leronlimab’s effects. CytoDyn has announced that the drug has been given to 49 patients and has kicked off a Phase 2 clinical trial, but has little evidence of efficacy in its press release beyond anecdotes – for example, when describing 11 people dosed with the drug in NY, the company says: “we believe we were able to save the lives of four patients.”
How has the stock market responded?
CytoDyn’s stock had lost 90% of its value since purchasing leronlimab in 2012, as the company missed deadlines and ran behind schedule for the drug. Since late November, however, CytoDyn’s stock has improved 1000% from 30 cents to a high of 3.73 on April 27. Last Thursday, Pourhassan filed to sell close to 5 million shares.
Bottom line it for me
It’s suspect when a CEO sells stock as they pump it up for investors, and especially so when there is such a lack of data.
Rapidfire
Despite the criticism the company has received for its role in the coronavirus pandemic, Carnival Cruise Line has announced that they’ll be reopening some of their ships before the end of this summer. Talk about cruisin’ for a bruisin’.
Contrary to earlier reports, new studies reveal that common blood pressure medications do not raise the risk of Covid-19 infection for the millions of people on these drugs.
This morning, Akebia Therapeutics announced positive data for its drug vadastutat in the treatment of anemia, a condition in which the body doesn’t have enough healthy red blood cells, in patients with kidney disease. Akebia’s tablet could provide a safe, convenient treatment alternative to the injectable anemia medicines that are currently used.
Investor Corner: Stemline Therapeutics
Stemline Therapeutics ($STML), the manufacturer of Elzonris, a drug for the treatment of a rare type of blood cancer, was bought for $677 million yesterday by Italy’s Menarini Group.
Stemline was purchased for $11.50 a share in cash with a further $1 a share if the company hits certain milestones in Europe. The sale represents an increase of 250% from Friday’s stock price.
Even though Stemline only made $43 million in its first full year of sales, Cowen analyst Boris Peaker believes it could max out at $250 million per year after gaining footing in Europe and reading out positive data in other types of cancer, like myelofibrosis and acute myeloid leukemia. Peaker wrote that these sales could drive a share value of $14-$20.
Stemline closed on Monday at $12.10.


