States around the US have begun to ease mask guidelines and social distancing restrictions. In New York, masks are no longer required indoors in most places, and indoor dining will return to 100%. We like to call this “Operation: Return to Precedented Times”.
So today, we’re covering some non COVID-19 related news, though it does come from one of the big vaccine players — Johnson and Johnson. The company just announced a positive data read out for their CAR-T therapy called cilta-cel for multiple myeloma. CAR-T therapy is one form of personalized medicine that many think can transform the future of cancer care. More on that story below.
Today’s Pulse is 600 words, or a 5 minute read.
If you like what you read, make sure to drag the email to your “primary” tab!
Clinical TrialsCAR-T therapy from J&J shows benefit for early stage multiple myeloma
The pulse:
This week, Johnson and Johnson announced that their trial for a CAR-T therapy called cilta-cel showed benefit for patients with early stage refractory multiple myeloma.
Multiple myeloma
Multiple myeloma (MM) is a type of blood cancer that can be devastating, partly due to the difficulty of achieving complete remission. Patients often undergo multiple modalities of treatment, only to have their myeloma come back each time. The five year survival rate is about 50%. Many patients will require a stem cell bone marrow transplant, which itself carries a mortality rate of about 30%.
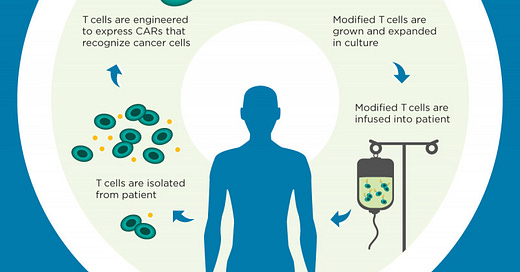
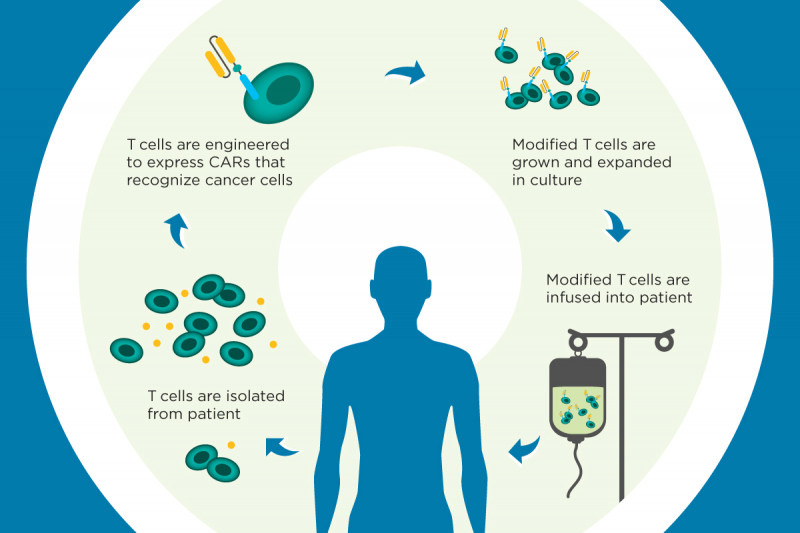
What is CAR-T therapy?
CAR-T therapy is an exciting form of personalized medicine that allows doctors to harvest a patient’s own T-cells (cells in the immune system that can fight against cancer) and engineer them specifically to better attack the tumor. Doctors draw blood from patients, filter out their T-cells and then modify them in a lab to target a specific protein their cancer produces. The designer T-cells are then returned to the patient to give their immune system a better chance of defeating the tumor.
CAR-T therapy was first approved by the FDA in 2017. Since then, there have been five approved CAR-T therapies. Johnson & Johnson are looking to make cilta-cel — which they have licensed from a Chinese biotech company called Legend Biotech — the sixth such approved therapy.
How CAR-T works Source: Memorial Sloan Kettering Cancer Center
What did the trial show?
The study looked at 20 patients with refractory multiple myeloma that had become unresponsive to a median of two prior treatments. These patients were given a one-time infusion of cilta-cel. The overall response rate was 95% and 75% of the patients had a complete response, meaning there were no detectable signs of myeloma remaining. Six months after infusion, none of the patients had suffered a relapse. It’s a strong showing for efficacy of the therapy.
Importantly, J&J and Legend Biotech were also able to show an improved safety profile from prior studies, which is often a concern with CAR-T therapy. Neurotoxicity — often manifesting as headache, confusion, seizures, or delirium — is a known side effect of all CAR-T therapies. Earlier studies of cilta-cel had revealed a number of neurotoxic events that could potentially hinder FDA approval. This latest study had fewer and less severe cases of neurotoxicity, partially due to stricter patient management strategies.
Will cilta-cel be FDA approved?
With the addition of this data showing improved safety and continued strong efficacy, the likelihood is looking better and better for J&J. The European Medicines Agency (EMA) just accepted the marketing application for cilta-cel. Johnson and Johnson sent in a rolling submission for cilta-cel to the FDA back in January. An approval decision is expected later this year.
Bottom line it for me:
Johnson and Johnson has read out promising data for its CAR-T therapy, cilta-cel. The data bodes well for its looming FDA approval and for patients with refractory multiple myeloma.